A groundbreaking new study has uncovered a surprising player in brain aging: a protein known as ferritin light chain 1 (FTL1). Researchers have discovered that reducing levels of this protein in aging mice led to remarkable improvements in brain function—suggesting that FTL1 may be a key driver of cognitive decline and a promising new target for therapies aimed at preserving brain health.
Ferritin light chain 1, or FTL1, is a component of ferritin, the primary protein responsible for storing and regulating iron in the body. Iron is essential for many biological processes, including oxygen transport and energy production in cells. However, when iron regulation goes awry, excess iron can accumulate in tissues—including the brain—leading to oxidative stress and cellular damage.
The new research, conducted at the University of California San Francisco (UCSF), reveals that FTL1 levels increase significantly in the brains of aging mice. This accumulation appears to disrupt iron homeostasis, contributing to inflammation, impaired neuronal function, and cognitive decline. Most strikingly, when scientists reduced FTL1 expression, the aging mice showed signs of brain rejuvenation.
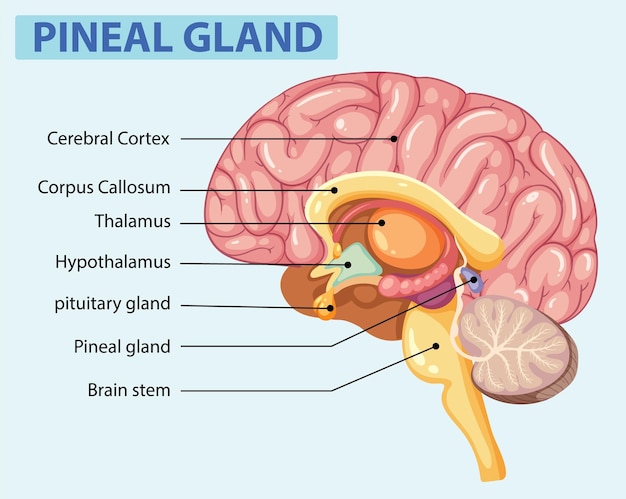
Changes in the brain associated with aging and iron accumulation
In the study, researchers used genetic techniques to reduce FTL1 levels in the brains of older mice. What they observed was nothing short of remarkable: the treated mice showed improved memory, enhanced synaptic plasticity (the brain’s ability to adapt and form new connections), and reduced signs of neuroinflammation.
Even more surprising, brain cells in these mice began to resemble those of much younger animals at the molecular level. This suggests that lowering FTL1 didn’t just slow aging—it actively reversed some of its effects. While these findings are still in the preclinical stage, they open the door to potential therapies that could one day help humans maintain cognitive function well into old age.
Iron accumulation in the brain has long been associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Excess iron can catalyze the production of reactive oxygen species (ROS), which damage proteins, lipids, and DNA in brain cells. Over time, this oxidative stress contributes to neuronal death and cognitive impairment.
FTL1 plays a critical role in this process. As a component of ferritin, it helps sequester iron to prevent toxicity. But paradoxically, elevated levels of FTL1 in aging brains may lead to dysfunctional iron storage, creating pockets of trapped iron that slowly leak and cause damage. The study suggests that too much FTL1 may actually impair the body’s ability to manage iron effectively in neural tissue.

Iron dysregulation and its impact on brain cells
While the results are promising, translating these findings to humans will require extensive further research. Scientists must first confirm whether FTL1 behaves similarly in the human brain and whether reducing its levels is safe and effective. One major concern is that iron regulation is vital throughout the body, and disrupting it systemically could have unintended consequences, such as anemia or impaired cellular metabolism.
Future studies may focus on developing targeted therapies—such as gene-silencing drugs or small molecules—that can selectively reduce FTL1 in the brain without affecting other tissues. Researchers are also exploring whether blood or cerebrospinal fluid levels of FTL1 could serve as biomarkers for brain aging or early neurodegeneration.
The discovery of FTL1’s role in brain aging could have far-reaching implications for treating age-related neurological conditions. If iron dysregulation is a common pathway in cognitive decline, then targeting proteins like FTL1 might offer a unified approach to preventing or slowing multiple disorders, including Alzheimer’s disease, Parkinson’s disease, and vascular dementia.
Moreover, this research shifts the focus from merely managing symptoms to potentially reversing the biological underpinnings of brain aging. It aligns with a growing trend in neuroscience: viewing aging not as an inevitable decline, but as a modifiable biological process.
Future therapies may target aging mechanisms like FTL1 to preserve brain health
The identification of FTL1 as a key player in brain aging marks a significant step forward in our understanding of cognitive decline. It offers not only a new therapeutic target but also a deeper insight into the biological mechanisms that govern brain health over time.
While we are still years away from human treatments based on this discovery, the study provides hope that one day, we may be able to delay—or even reverse—memory loss and maintain mental sharpness throughout life. As research continues, FTL1 could become a cornerstone in the fight against age-related brain diseases.
The journey to unlock the secrets of brain longevity has taken a bold new turn—and FTL1 may be leading the way.

Health

Health

Health

Health

Health

Health
Health

Health

Health

Health

Health

Wellness

Health

Fitness

Health

Health