The global response to the Covid-19 pandemic included one of the fastest vaccine development efforts in history. Messenger RNA (mRNA) vaccines, a groundbreaking technology at the forefront of this effort, were rolled out with unprecedented speed and scale. While these vaccines have been credited with reducing severe illness and hospitalizations, they have also sparked ongoing scientific and public debate—particularly around long-term safety and regulatory oversight.
Recent reports indicate that a number of individuals involved in shaping vaccine policy or advising health authorities have expressed reservations about the safety of mRNA-based Covid-19 vaccines. Among them, at least three notable figures have publicly questioned aspects of the technology, its deployment, and the speed of regulatory approval. Their concerns, while not representative of the broader scientific consensus, highlight the importance of transparency and continued monitoring in public health initiatives.
Unlike traditional vaccines that use weakened viruses or viral proteins, mRNA vaccines work by instructing cells to produce a harmless piece of the spike protein found on the surface of the SARS-CoV-2 virus. This triggers an immune response, preparing the body to fight the actual virus if exposed. The technology was decades in the making but saw its first widespread use during the pandemic.
Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) granted emergency use authorizations based on robust clinical trial data showing high efficacy and acceptable short-term safety profiles. Millions of doses were administered globally within months, marking a historic public health achievement.

Despite broad scientific support, some experts have raised concerns about the long-term effects of mRNA vaccines, particularly given their rapid deployment and limited post-authorization surveillance at the time of rollout. These concerns are not centered on rejecting vaccines outright, but rather on advocating for more rigorous, independent evaluation and open discussion of potential risks.
According to recent reports, a list of individuals being considered for advisory roles in vaccine policy includes at least three who have previously questioned the safety or regulatory process surrounding mRNA Covid-19 vaccines. Their inclusion has reignited debate about scientific diversity, risk communication, and the balance between urgency and caution in public health decision-making.
Further fueling the discussion, internal records have revealed instances where top regulatory officials overruled recommendations from scientific advisory panels. In one documented case, a senior FDA official rejected the input of agency experts and made unilateral decisions to limit or expand vaccine use under emergency provisions. Such actions, while legally permissible during public health emergencies, have prompted calls for greater transparency and accountability.
Critics argue that bypassing consensus-based scientific review—even in urgent situations—can erode public trust. Proponents, however, maintain that decisive leadership was necessary to respond to a rapidly evolving crisis. The tension between these perspectives underscores the complexity of balancing speed, safety, and democratic oversight in health policy.

One area of agreement across the scientific spectrum is the need for robust post-market surveillance. Vaccines, like all medical interventions, carry potential risks, and rare side effects may only become apparent after millions of doses are administered. Systems like the Vaccine Adverse Event Reporting System (VAERS) in the U.S. play a critical role in detecting signals that warrant further investigation.
Long-term studies continue to assess the durability of protection, the impact of booster doses, and any delayed adverse events. Independent research, transparent data sharing, and international collaboration remain essential to building a comprehensive understanding of mRNA vaccine safety and efficacy.
Public confidence in vaccines depends not only on scientific evidence but also on how that evidence is communicated. Dismissing concerns outright can alienate individuals seeking reassurance, while amplifying unverified claims can lead to unnecessary fear. A balanced approach—one that acknowledges uncertainties while emphasizing the overwhelming benefits—can foster more informed decision-making.
Including diverse voices in advisory panels, even those who have expressed skepticism, may help restore trust by demonstrating that dissenting views are heard and evaluated. However, such inclusion must be grounded in scientific rigor and ethical responsibility, ensuring that policy recommendations remain evidence-based.

The development of mRNA vaccines represents a milestone in medical science with potential applications beyond Covid-19, including cancer therapies and treatments for other infectious diseases. As this technology evolves, so too must the frameworks for evaluating its safety, ethics, and societal impact.
The current debates are not a sign of scientific failure, but rather a reflection of science in action—self-correcting, adaptive, and responsive to new information. Ensuring that regulatory processes remain transparent, inclusive, and accountable will be key to maintaining public trust in future health interventions.
As we move forward, the lessons learned from the pandemic—both in terms of scientific innovation and public communication—will shape how we respond to health challenges for years to come.

Health

Health

Health

Health
Health
Health

Health
Health
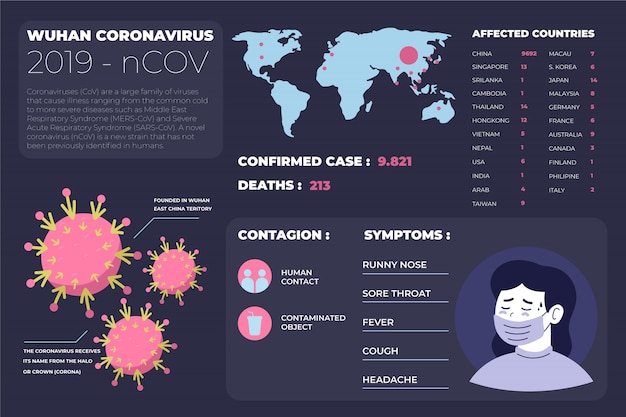
Health

Health

Health

Health

Health

Fitness

Health

Health