In a surprising development that has sparked widespread discussion, new reports suggest that the U.S. Food and Drug Administration (FDA) is reevaluating its long-standing position on the safety and effectiveness of certain vaccines. While the agency has historically maintained a firm stance supporting immunization programs, recent internal discussions and leadership changes have brought new scrutiny to its processes and public messaging.
Though no official reversal has been issued, sources within the agency indicate growing debate among senior officials about the long-term data, transparency, and risk-benefit profiles of some widely administered vaccines. This shift comes at a time of heightened public skepticism and increasing demand for independent review of clinical data used in emergency use authorizations and full approvals.
Recent changes in key leadership roles at the FDA have coincided with a broader reexamination of vaccine policy. As new officials take on responsibilities in the agency’s Center for Biologics Evaluation and Research (CBER), there has been a push for more rigorous data transparency and independent verification of clinical trial results.
One notable development is the increased emphasis on reviewing raw clinical data, rather than relying solely on summaries provided by pharmaceutical companies. This move aligns with long-standing calls from scientists and public health advocates for greater openness in the regulatory process.

FDA headquarters in Silver Spring, Maryland — a focal point of evolving vaccine policy
For years, critics have argued that the FDA’s vaccine approval process lacks sufficient public access to underlying trial data. Now, internal discussions reflect a growing acknowledgment that restoring public trust requires more than official statements — it demands verifiable evidence.
Some senior officials have reportedly expressed concern that the agency’s rapid authorization of vaccines during the pandemic may have bypassed traditional safeguards. While these measures were justified under emergency conditions, there is now debate about whether long-term monitoring has kept pace with the speed of initial rollout.
The FDA has begun releasing select datasets in response to Freedom of Information Act (FOIA) requests, though advocates say the process remains slow and incomplete. Full access to data on adverse events, efficacy over time, and comparative outcomes across age and risk groups is still limited.
When regulatory agencies express uncertainty, it can have significant implications for public confidence. The FDA has not stated that vaccines are unsafe or ineffective overall, but the acknowledgment that some conclusions may need reevaluation marks a notable shift.
This does not mean that vaccines should be dismissed. On the contrary, vaccines remain one of the most effective tools in preventing infectious disease. However, the FDA’s willingness to question its own assumptions reflects a maturing scientific process — one that prioritizes ongoing evaluation over static conclusions.
Experts emphasize that uncertainty is not the same as risk. All medical interventions carry potential side effects, and the key is balancing benefits against known and emerging risks. The current dialogue within the FDA suggests a more nuanced approach is emerging — one that considers individual risk factors, variant evolution, and waning immunity over time.

Ongoing research continues to inform vaccine safety and efficacy assessments
As news of internal FDA discussions spreads, there is a risk of misinterpretation. Some groups may use the agency’s caution as evidence of broader failure, while others may dismiss concerns outright. Navigating this landscape requires clear, fact-based communication from health authorities.
Health officials stress that scientific debate is healthy and necessary. Revisiting conclusions in light of new data is a sign of strength, not weakness. However, they also warn that misinformation can spread faster than context, potentially undermining vaccination efforts.
The FDA is expected to release updated guidance on vaccine evaluation in the coming months. This may include revised recommendations for booster intervals, expanded monitoring for rare adverse events, and greater emphasis on personalized vaccination strategies.
Additionally, the agency is exploring ways to improve real-time data collection through electronic health records and national surveillance systems. These tools could help detect patterns more quickly and support more agile decision-making.
Ultimately, the goal is to build a regulatory framework that is both scientifically rigorous and responsive to public concerns. Transparency, accountability, and independent review will be key pillars of this evolving approach.
The FDA’s current reevaluation of vaccine safety and effectiveness reflects a broader trend in science: the recognition that knowledge is dynamic. What was understood in 2020 may require refinement in 2024 as more data becomes available.
Rather than signaling a reversal, this moment represents an opportunity to strengthen public trust through openness and scientific integrity. As the agency continues its work, the importance of clear communication, independent oversight, and evidence-based policy has never been clearer.
The path forward will require collaboration between scientists, regulators, healthcare providers, and the public — all working toward a shared goal of safer, more effective health interventions.

Health

Health

Health

Health
Health
Health

Health
Health
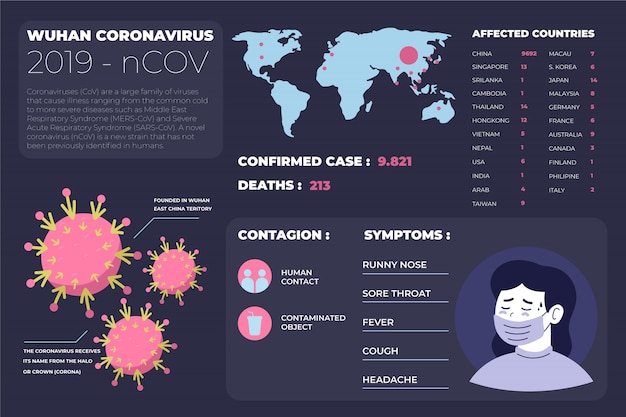
Health

Health

Health

Health

Health

Fitness

Health

Health